Zhong lab in Medical Research Institute Reports the role of USP2a in promoting metastasis by facilitating TGF- triggered signaling
On February 27, 2018, Dr Bo Zhong and colleagues published their latest research entitled” USP2a supports metastasis by tuning TGF-b signaling” in Cell Reports.
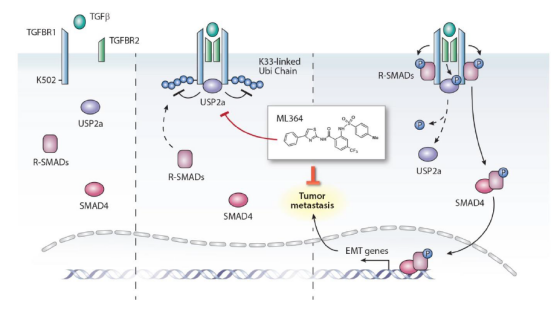
Atypical symptoms of cancers, the cancer cells are often found to metastasize into nearby lymph nodes or tissues and colonize in the same organs or distant parts of the body along with the diagnosis of cancers, and such metastasis is believed to be responsible for up to 90% of solid tumor-associated deaths. It has been demonstrated that TGF-b induces EMT and thereby promotes metastasis. TGF-b binds to the heterodimeric type II and type I TGF-b serine/threonine kinase receptors (TGFBR2 and TGFBR1) and initiates a signaling cascade through phosphorylation of SMAD2 and SMAD3 known as receptor-regulated SMADs (R-SMADs) The phosphorylated R-SMADs disassociate from the receptors, bind to SMAD4 and enter into nucleus to regulate gene expression. But the regulatory mechanisms of how R-SMADs were recruited and disassociated are poorly understood. Here the author found that USP2a interacts with TGFBR1 and TGFBR2 upon TGF-b stimulation and removes K33-linked polyubiquitin chains from Lys502 of TGFBR1, promoting the recruitment of SMAD2/3. Simultaneously, TGFBR2 phosphorylates Ser207/Ser225 of USP2a, leading to the disassociation of SMAD2/3 from TGFBR1. Depletion or pharmacologic inhibition of USP2a dampens TGF-b-triggered signaling and metastasis. Our findings have characterized an essential role of USP2a as a potential target for treatment of metastatic cancers.